Research
1. Muon Chemistry
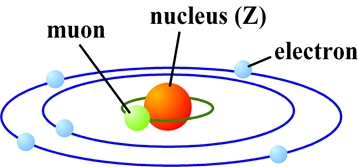
A muon, one of the elementary particles, that has the same negative charge as an electron and 207 times larger mass than that of an electron. When a muon introduces in a substance, the muon make a muon atomic orbit around a nucleus, and forms an “exotic” atomic system called a muonic atom (Figure 1).
Because of large mass of a muon, a radius of atomic muon orbit is small compare to that of electron orbit and the muon exists close to the nucleus. However, the muon capture process in an atom (muonic atom formation process) is strongly influenced by the chemical forms of muon capturing atom. We are studying on such chemical effects on muon capture.
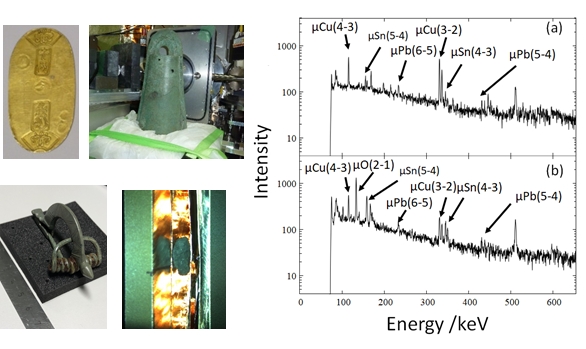
After the muonic atom formation, characteristic muonic X-rays are emitted by the muon inter-orbital transition. Since the energy of muonic X-ray is unique by each element, elemental analysis is possible by measuring muonic X-rays. The high energy properties of muonic X-rays enable us “non-destructive” elemental analysis including light elements. We are developing a novel elemental analysis method using muon and applying this method for valuable samples, such as cultural properties and samples recovered from asteroids (Figure 2).
We are also studying various research topics related to muon, fundamental interactions between muons and materials, muon lifetime measurement in matter, and so on. Let's work together to establish a new chemical research field, “elementary particle chemistry” !
2. Environmental Radiochemisty
In 2011, nuclear accident at the Fukushima Daiichi Nuclear Power Plant of Tokyo Electric Power Company was occurred, and a large amounts of radioactive nuclides were released into the environment. In this accident, all of the radioactive nuclides existed in the reactor were never emitted and distribution of radioactive nuclides in the environment was heterogeneous. Why ?
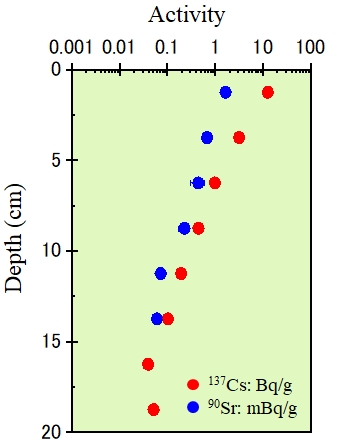
The released ratio, distribution in the environment and migration process of each radioactive nuclide are strongly influenced by the properties of the elements (melting point, boiling point, oxidation state, possible chemical form, etc.), meteorological conditions, and properties of natural environment such as pH. We are studying on the details on circumstances in the reactor at the accident and present status of radioactive nuclides (Figure 3).
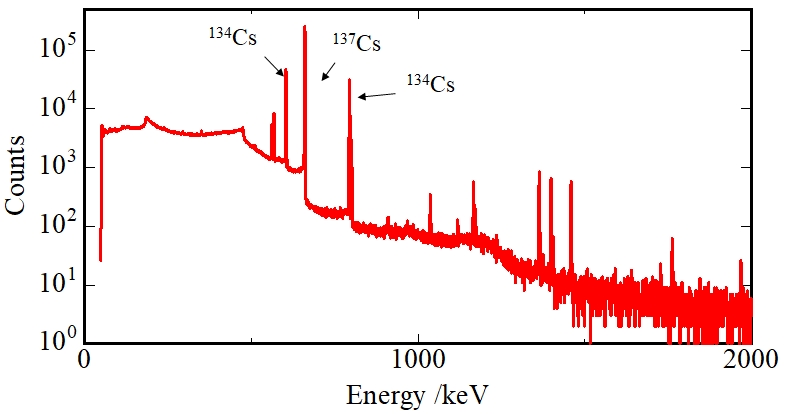
Although large amounts of radioactive nuclides were surely released by this accident, the total mass of each element was up to a few kg and the nuclides were spread in large area. Therefore, concentration of radioactive nuclides in the environment are very low; less than pico-moles (10-12 mol, about 1010 atoms). We can clarify the presence of these low concentration elements, such as cesium, strontium, and plutonium, using an extremely sensitive detection probe, radiation (Figure 4).
Only radiochemist can detect such low concentration elements. Let’s investigate migration of elements in the environment by radiochemical methods.